
Neo BioMed Services has been engaged, since 2006, in providing expert technical, regulatory compliance and business support services to Small and Mid-Size Pharmaceutical, Biotechnology, Stem Cell, Biologics, Diagnostic, Medical Devices and allied Health Industries including biodefense industries.
Neo BioMed Services believes in quality of service par excellence and is joining hands with national and international consulting agencies in providing support to solutions meeting the strictest regulatory norms. Our associates are proficient in development, implementation and management of diversified manufacturing and supply chain projects involving the integration of scientific principles, regulatory aspects and business requirements and management.
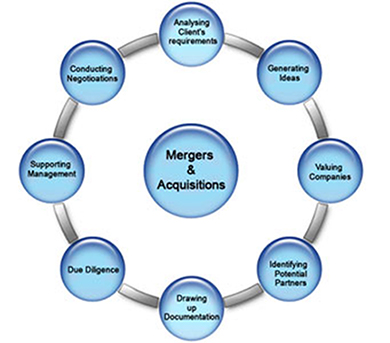
Neo BioMed Services assist the stakeholders to develop cGMP compliant quality products from concept to commercialization, effective Technology Transfer, regulatory submissions, liaisoning and necessary government approvals. Services are focused on cGMP regulated clinical or commercial Active Pharmaceutical Ingredients (APIs), Rx and OTC drug products, Diagnostic products and tools or Medical Devices.
• Based in New Delhi, India
• Service Providers
• Consultants
NeoBioMed Services, founded in May 2006, is a privately held partnership company based in New Delhi, capital city of India. The company is led by a management team headed by its Chief Executive Officer Dr. Brij M. Gandhi, (LINK) a scientist and a technocrat with more than 52 years of experience; 21.5 years in clinical research sciences and services; over 17 years of experience as Technocrat with the Department of Biotechnology, Ministry of Science and Technology, Government of India; and 7 years experience post retirement as Advisor / Director / Consultant to a number of Universities, Institutions, Industries and Industry Organizations. As Adviser to the Government of India, Dr. Gandhi headed the Departments of International Cooperation, Medical Biotechnology, Infrastructure, Animal Biotechnology and Aquaculture.
View More